31+ calculating the ph of a buffer
Web Calculate the pH of a buffer solution formed by adding 2000 cm 3 of 010 moldm-3 NaOH to 4000 cm 3 of the weak acid HX which has a concentration of 020 moldm-3 and a Ka. Another example of calculating pH of a soluti.

Table 9 From Calculation Of The Ph Of Buffer Solution Of 2 N Morpholino Ethanesulfonic Acid Mes From 5 C To 55 C Semantic Scholar
If a buffer solution contains 0200 M formic acid pKa 375 p K a 375 and 0200 M sodium formate what is the pH of this solution.

. Web For comparison calculate the pH after 10 mL of 010 M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 474 eg. The volume of the final solution is 101 mL. All in one place.
Web The pH scale pH is a numeric scale used to define how acidic or basic an aqueous solution is. Fill concentrations of each acid and base. PKa p K a is 93.
Web How to use the Henderson-Hasselbalch equation to calculate the pH of a buffer. C H X 3 C O O H N a O H C H X. Enter the desired final volume and concentration and click Calculate Mass The exact.
Web Please follow following steps to determine pH of buffer solution. So lets compare that to the pH we got in the previous problem. Select the acid base combination of buffer solution.
List the values you are given. Web Calculate the pH in a buffer prepared from pu50 mL 030 M formic acid ceHCOOH and pu30 mL 040 M sodium formate ceHCOONa. Web Calculating pH of buffer.
From the calculation above the pH of buffer solution is 738. K a 176 10 5 m o l L. It commonly ranges between 0 and 14 but can go beyond these.
For the buffer solution just starting out it was 933. Now lets check our answer to see whether its reasonable. Ok so I know acetic acid will react with sodium hydroxide do form sodium acetate.
1 begingroup The answer keys method is certainly valid given that the system is buffered. Web What is the p H. A 18 10 5-M solution of HCl.
The counteracting abilities only work for certain values of pH after which the solution would experience. Web The operating range of a pH buffer solution is pretty limited. Web Example of calculating the pH of solution that is 100 M acetic acid and 100 M sodium acetate using ICE table.
Web If a buffer solution contains 10 M HCN H C N pKa 93 and 01 M CN C N what is the pH of this solution. Web The empirical formula pKa buffer pH range formula weight and product list will appear. It ranges from 0 to 14 with 7 being neutral and values above 7 indicating alkaline solutions and.
1069 6 6 silver badges 10 10 bronze badges endgroup 4. Web Calculating the pH of a Buffer. Calculate the pH of an acetate buffer that is a mixture with 010 M acetic acid and 010 M sodium acetate.
Determine the mathrmpH of the solution resulting when pu100 cm3 of pu050 mol dm-3 ceCH2ClCOOH is mixed with pu200. Web The pH is equal to 925 plus 12 which is equal to 937. Web The pH scale is used to measure the acidity or basicity of a solution.
Lesson Plans and more.

Comparison Of The Measured And Calculated Ph Values For Ammonium Buffers Download Scientific Diagram

Buffer Ppt Download

4 Ph Values Of Buffer Solutions At The Reaction Temperatures Download Table

Calculate Ph Of Buffer After Adding Strong Base Youtube

How To Calculate Ph Of Buffer Solutions Sciencing

How To Calculate Ph Of Buffer Solutions Sciencing

How To Calculate The Ph Of A Basic Buffer And Calculate The Ph Of Buffers As They Are Titrated Youtube

How To Calculate The Ph Of A Buffer Solution Quora

Solved You Need To Make A Buffer At Ph 6 5 Using Any Chegg Com

Acids And Bases Ph And Buffers Ppt Video Online Download

Find The Ph Of A Buffer Solution Youtube
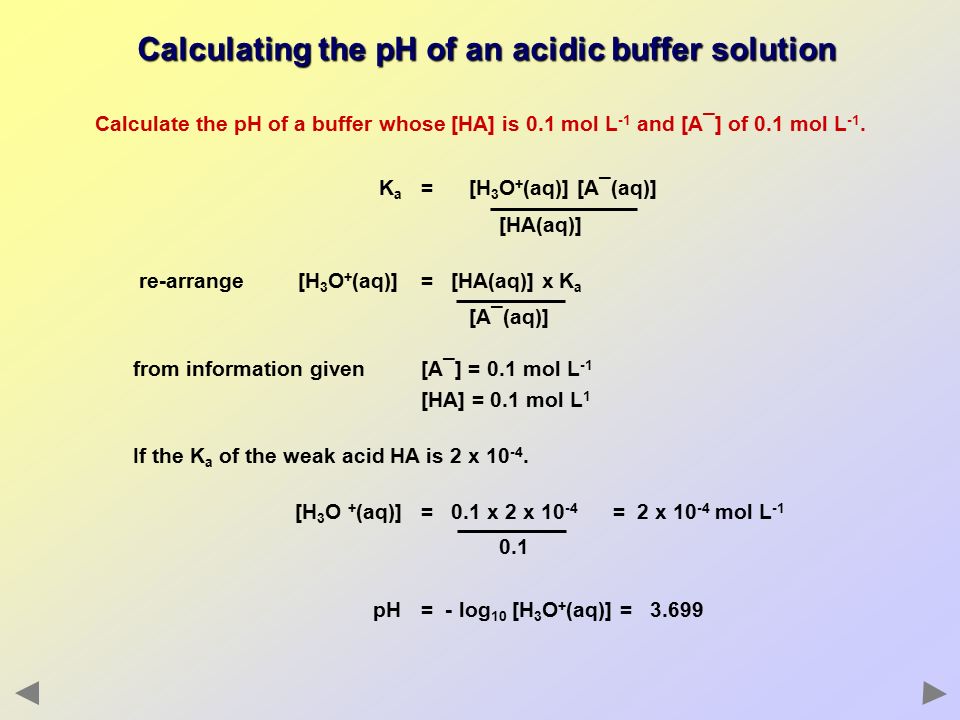
Buffer Solutions Ppt Download

Calculating The Ph Of Buffer Solutions Youtube
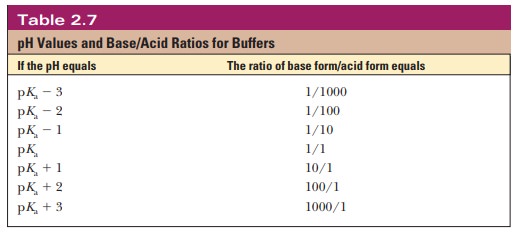
How Do We Choose A Buffer
Buffer Ph Calculator
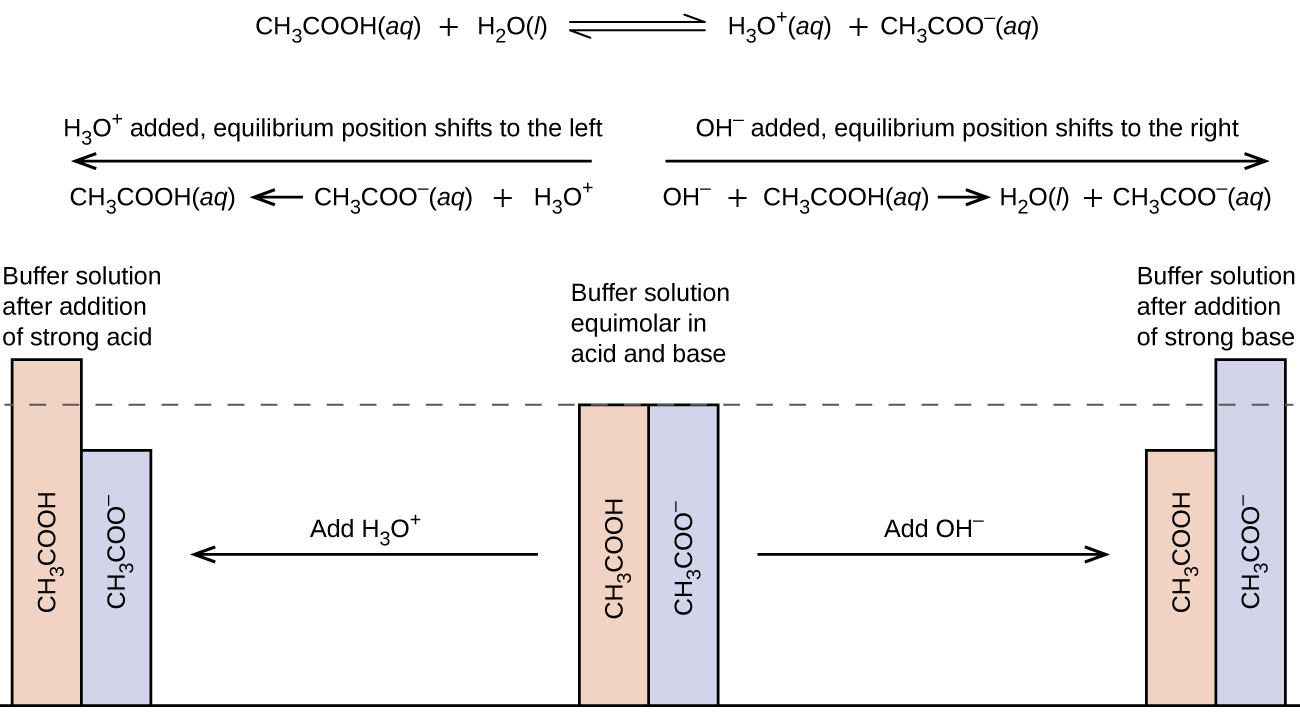
M16q1 Defining A Buffer Calculating The Ph Of A Buffer Solution Chem 103 104 Resource Book

Calculate Ph Of Buffer Solution